Screening
Once we have the results of all your tests, we will let you know if you are suitable for the trial. One of our team will contact you to re-confirm the dates and times you need to be at our clinical unit.
If any of the results of your test are of concern to our doctors, this will be followed up directly with you. If you are not suitable for the study you were interested in, it is usually because you do not fit the criteria for that particular trial. Don’t worry, there are generally other trials that we can offer you straight away. Not all volunteers invited into clinic at the start of the trial will be dosed. You may be a reserve volunteer (alternate), as we need to ensure the trial is completed on time. We usually invite additional volunteers on standby in case of illness or non-attendance. In each case, the medical staff make the final decision on who will or will not be dosed, impartially and based on medical assessment and all reserve volunteers are reimbursed for their time and inconvenience. Once the trial has started, you are still free to leave the trial at any point, without giving a reason. However, you will only be paid for the days you completed.
You will need to bring a valid photo ID (Driver’s License, State ID, or Passport) to your appointment. The screening appointment is an evaluation to determine if you qualify for study participation. You will receive a detailed explanation of the study specific informed consent document. Once you have signed the document, you will have a thorough medical examination at no cost to you. The medical examination includes medical history, height, weight, blood pressure, pulse and temperature measurements, electrocardiogram and physical exam. A blood sample and urine sample will also be collected for laboratory tests including a screen for drugs of abuse, hepatitis and HIV screen, and pregnancy test (if female of childbearing potential). Some clinical trials require additional tests, which would be listed in the informed consent document.
Additional information:
- A full screening visit can typically take up to 4 hours
- Contraception – we will have told you if there are specific contraception requirements that should be in place before you attend for a screening. These may need to continue during and post-trial. In most cases, it is important that you and your partner are using two suitable methods of contraception whilst you are in the study and for a period of time afterwards. If this is not in place prior to your screening appointment, you may not be allowed to participate in the trial
- Avoid poppy seeds 48 hours before the screening as they can make you test positive for certain drugs of abuse
- Do not drink alcohol at least 48 hours before screening – this can sometimes be longer so ask our recruitment team when making your appointment
- Avoid exercising 72 hours before screening – physical activity can influence out-of-range blood test results, blood pressure and ECG results
- Some screening visits need you to have fasted for a period of time before you attend – the recruitment team will tell you about this when they book your appointment
- Ladies are advised not to wear dresses for the screening appointment as this can make it difficult to have an ECG test
Your stay
Yes, all meals will be provided. We have a catering service that meets study requirements. . You are not permitted to bring your own food into the unit. There are restrictions on what you can eat and drink on some trials, or there may be a specific diet that you have to follow during the trial. In all cases, volunteers must eat the meals provided at the designated times for the study and are not allowed to share their food with others during their stay in clinic.
Not on your own. If it is a long stay and the clinical trial sponsor allows it, we try to arrange walks around the building. You would always have staff members with you to ensure your safety. If you decide to leave the unit during a residential stay without prior agreement, you will be withdrawing yourself from the trial.
- Clothes for your stay – although we do have a washer and dryer on each unit, you will only be allowed to do laundry at designated times
- Sensible nightwear - no night dresses or night gowns
- Comfortable footwear – for health and safety reasons, you must have something on your feet at all times whilst in the clinic; no flip flops are permitted
- Washing essentials (soap/shower gel, toothbrush, toothpaste etc.) – no mouthwash though as it may contain alcohol that can affect the results from the trial
- Towels
- Some volunteers bring their own pillow with them
- Anything you might want to do while you are in the clinic e.g. laptop, books, iPad, earphones, and college work.
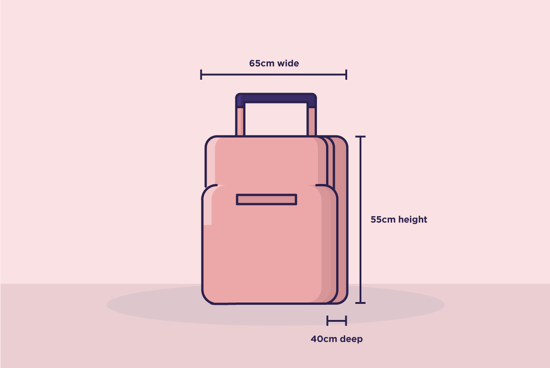
When you are packing your things to come on to a trial, please take a moment to check the size of your bag. We have very limited storage space for bags in the clinic and cannot accommodate any bags larger than a standard cabin flight case. When you are in the clinic your bag must be stored safely with no trailing straps etc. Safe storage of your bag is your responsibility.
We will tell you in advance what time you need to come in and other relevant information, such as if you need to fast beforehand. We normally ask that you come in the day before you will be dosed with the study medicine. Once all the volunteers have arrived, one of the nurses will talk to you all and answer any questions. There are often more medical tests to confirm you are still okay to take part before you can be given the study medicine.
Your safety
Registering your interest in a trial does not mean you have to complete the whole process. You can change your mind about taking part in a trial at any time, even once it has started.
The majority of our volunteers do not experience any side effects. However, if they do, the most common side effects are feeling sick, dizziness and headaches. We will monitor you closely throughout the trial and if you do experience any side effects, we will take appropriate action to look after you.
All medicines can have side effects, even those already available. Sometimes volunteers do experience these, but we have doctors and nurses on site who constantly monitor any reaction to make sure you are safe at all times. You will be given an informed consent form, which lists possible risks and side effects you may experience during the study. However, it is possible that any test medicine may have side effects that are not yet known.
Before treatments can be tested on people, they will already have undergone extensive investigations in a laboratory for many years. If the results from this testing is positive, the next step is to seek approval for a trial to begin in healthy volunteers. An independent ethics committee looks at all aspects of a new trial and will act to safeguard the rights, safety and well-being of volunteers who wish to take part.
Your compensation
Payments made to you for taking part in a clinical trial will be reported as income to the United States Internal Revenue Service (IRS). No deductions for state or federal withholding will be made. It will be your responsibility to report compensation.
Your trial may involve follow up appointments or phone calls that will happen after you have completed your residential stay in clinic and you will be aware of this before you take part in the trial. Payments are made via a pre-paid debit card and are usually issued within 7 days of study completion.
Payments made to you for taking part in a clinical trial will be reported as income to the United States Internal Revenue Service (IRS). It will be your responsibility to report compensation received on state and federal tax returns and to pay any taxes due on your compensation.
After your trial
We always aim to provide the best possible service to all our volunteers but if you do have a complaint or concerns over the service you have experienced then we would like to know about this. In the first instance we would encourage you to raise your complaint/concern directly with one of our managers at the time the problem occurs. We would always prefer to try to resolve any issues with you at the time. However, if you feel that you are not able to do this or you feel that your complaint/concern cannot be dealt with at this level then please put your complaint in writing to the Senior Director of Operations.
To help us deal with your complaint as effectively as possible please include as much detail as you can. We will acknowledge receipt of your complaint and will provide you with details of when a full response can be expected, in most cases no more than two weeks from the date of receipt of the complaint.
The usual rest period before participating in another study is 30 days. However, some clinical trials require up to a 90 day period between study participation.
When you leave the clinic, you will be given a trial participation card that includes contact information for a doctor at Quotient. It is important you keep this information on hand and do not hesitate to contact us should you have any concerns or worries once you get home.